Proven to deliver results
for both adults and
children in a 1-year trial2
Demonstrated favorable results in both pharmacodynamic and functional measures1
When it comes to efficacy, Lamzede has favorable results in several important disease categories.1
Trial 1 (rhLAMAN-05) results show the benefit of Lamzede for patients with alpha-mannosidosis, especially over time and in pediatric patients2
Studied in an even distribution of pediatric and adult patients1
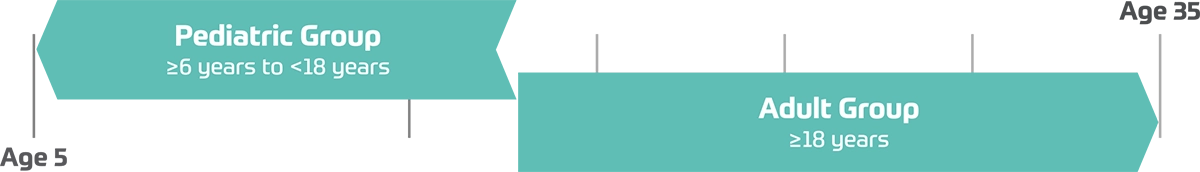
Patients were 6-17 years old (pediatric) and 18-35 years old (adult), with confirmed diagnosis of alpha-mannosidosis.
See eligibilitycriteria
Lamzede achieved significant pharmacodynamic response1
Patients receiving Lamzede showed a favorable response in both pharmacodynamic and functional measures.
View co-primaryendpoints
Favorable results in prioritized secondary endpoints1
Patients receiving Lamzede showed favorable results in endurance walking and pulmonary function.
View prioritizedsecondary endpoints
Study design
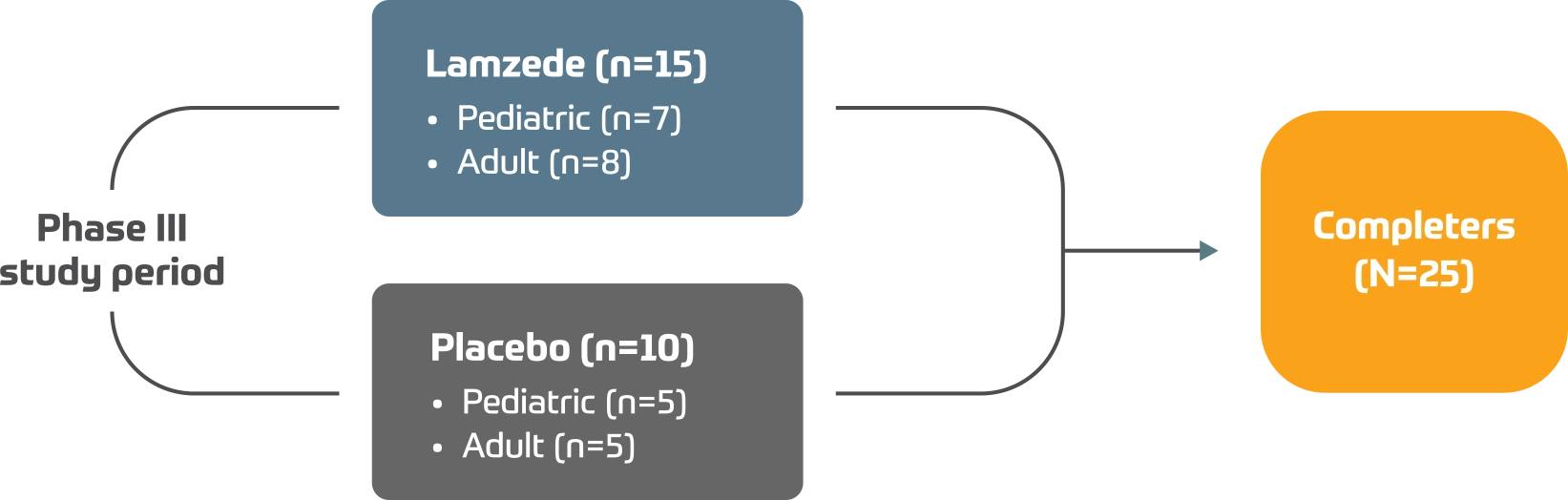
Trial 1 (rhLAMAN-05) was a Phase III, international, multicenter, double-blind, randomized, placebo-controlled parallel-group trial which assessed Lamzede in patients with alpha-mannosidosis.2
Efficacy and safety assessed in 25 patients with this ultra-rare disease.2

Eligibility criteria included confirmed α-mannosidase activity and no history of bone marrow transplantation2
Inclusion criteria1,2
- Confirmed α-mannosidase activity (<10% of normal activity in blood leukocytes)
- Ability to cooperate physically and mentally in trial assessments
Exclusion criteria2
- Presence of known chromosomal abnormality and syndromes, other than alpha-mannosidosis
- Inability to walk without support (the use of walking aids/wheelchair for partial support and longer distances was permitted)
- History of bone marrow transplantation
- Any psychotic disease, active or in remission
- Total immunoglobulin E (lgE) >800 IU/mL
Eligibility was not limited by motor performance at baseline.
Demonstrated favorable response vs placebo in both co-primary endpoints.1
Co-primary endpoints2
- Serum oligosaccharides change from baseline to week 52
- 3-minute stair-climbing test (3MSCT)
Reduction in a key marker of impaired cellular function1
- Improvements at 6 months that continued at 1 year
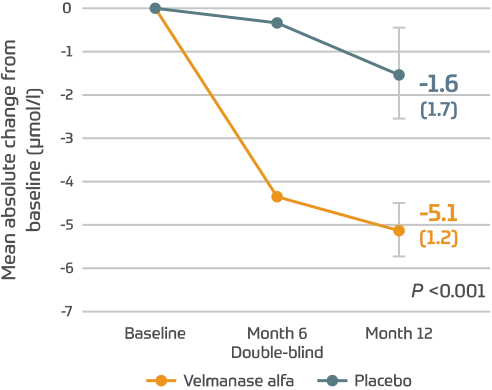
Serum oligosaccharides2,3
(95% CI: -4.4, -2.6)
| Lamzede | Placebo | |
|---|---|---|
| Baseline (SD) | 6.8 (1.2) | 6.6 (1.9) |
| Mean relative change % (SD) |
-75.8% (11.2) |
-20.3% (24.0) |
Lamzede reduced oligosaccharides by 75.8%.1
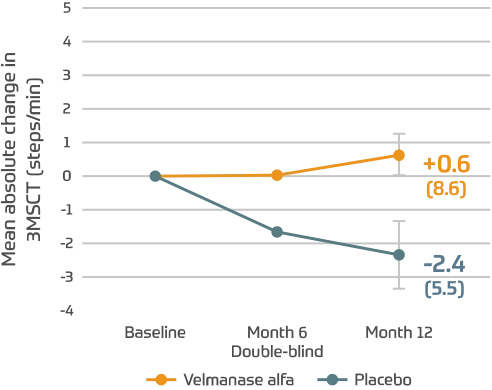
Maintained endurance climbing stairs, as measured by the 3MSCT1
- Patients who received placebo declined over time
3MSCT2
(95% CI: -3.8, 9.1)
| Lamzede | Placebo | |
|---|---|---|
| Baseline (SD) | 52.9 (11.2) | 55.5 (16.0) |
| Mean relative change % (SD) |
+0.5% (16.1) |
-3.6% (13.1) |
Lamzede patients showed a slight increase in steps per minute after 1 year.1,*
*Differences in 3MSCT were not statistically significant.

Trial 1 (rhLAMAN-05) prioritized secondary endpoints showed favorable results in measures of functional capacity vs placebo1
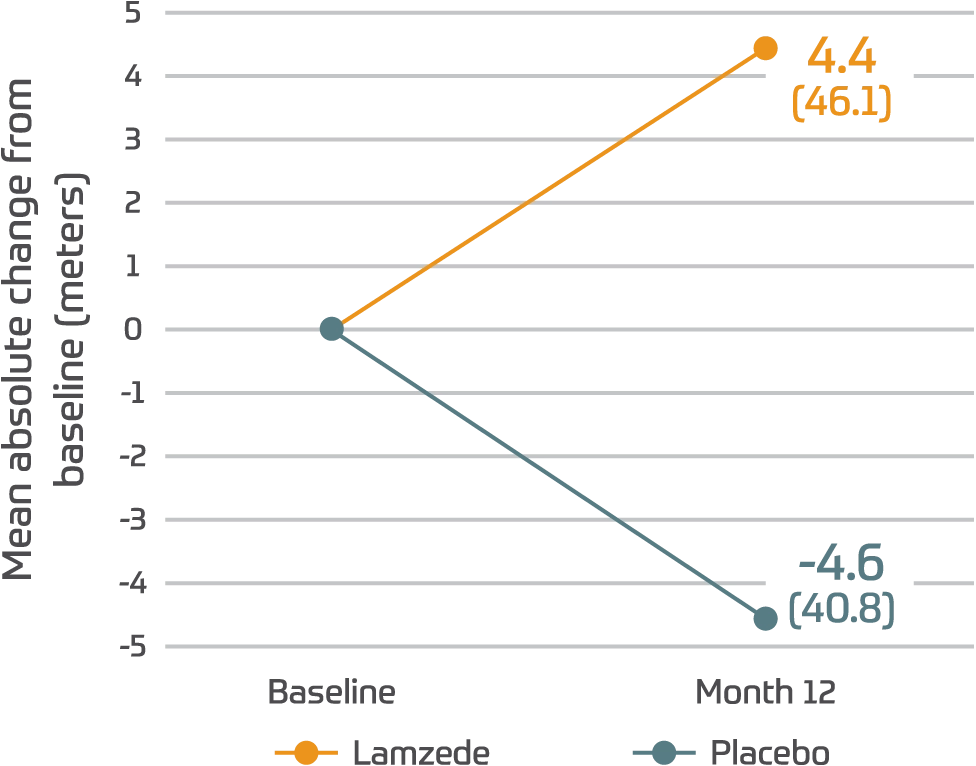
6MWT1
(95% CI: -30.7, 45.5)
| Lamzede | Placebo | |
|---|---|---|
| Baseline (SD) | 459.6 (72.3) | 465.7 (140.5) |
| Mean relative change % (SD) |
+1.2% (9.8) |
-0.8% (10.8) |
†Differences in 6MWT were not statistically significant.
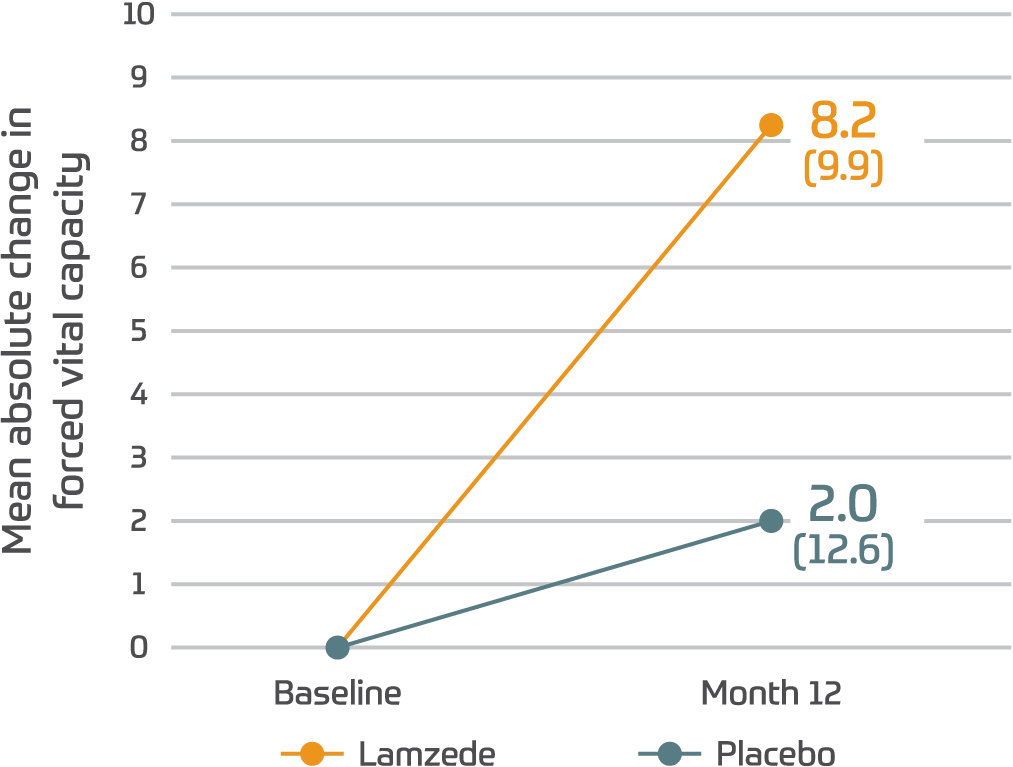
FVC%1
(95% CI: -5.0, 16.1)
| Lamzede | Placebo | |
|---|---|---|
| Baseline (SD) | 81.7 (20.7) | 90.4 (10.4) |
| Mean relative change % (SD) |
+11.4% (13.1) |
+1.9% (15.4) |
‡Differences in FVC test were not statistically significant.
†Differences in 6MWT were not statistically significant.
‡Differences in FVC test were not statistically significant.