Lamzede showed pharmacodynamic
and functional improvement over
the long term—up to 4 years2

In the Trial 3 integrated analysis (N=33), investigators pooled the cumulative databases from Lamzede Phase I, II, and III trials in patients with alpha-mannosidosis2,*
- 20 male and 13 female patients aged 6 to 35 years old (14 adults, 19 pediatric) received Lamzede weekly for a mean exposure of 89 weeks in adult patients and 155 weeks in pediatric patients
- Overall mean exposure was 29.3 (15.2 SD) months

Phase I and II
(follow-up to 48 months)
(follow-up to 48 months)


Phase III
(active for 12-36 months)†
(active for 12-36 months)†

Phase III (placebo for 12 months and then active to month 36)‡

Phase I, II and III§
(only 12 months data)
(only 12 months data)
Lamzede delivers sustained results over time.2
Co-primary endpoints2
- Serum oligosaccharides change from baseline to last observation
- 3-minute stair-climbing test (3MSCT) from baseline to last observation
Serum oligosaccharides2
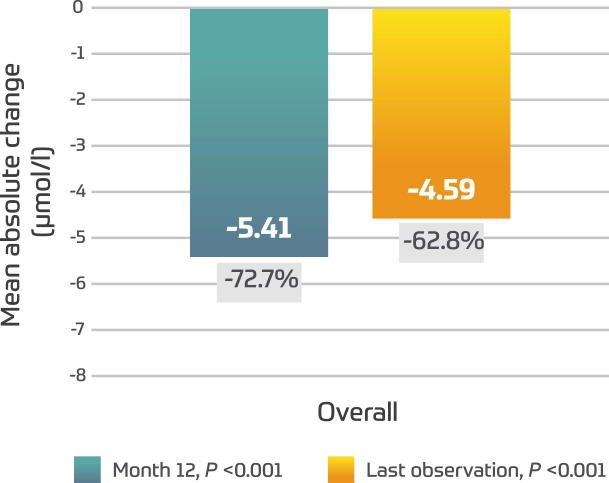
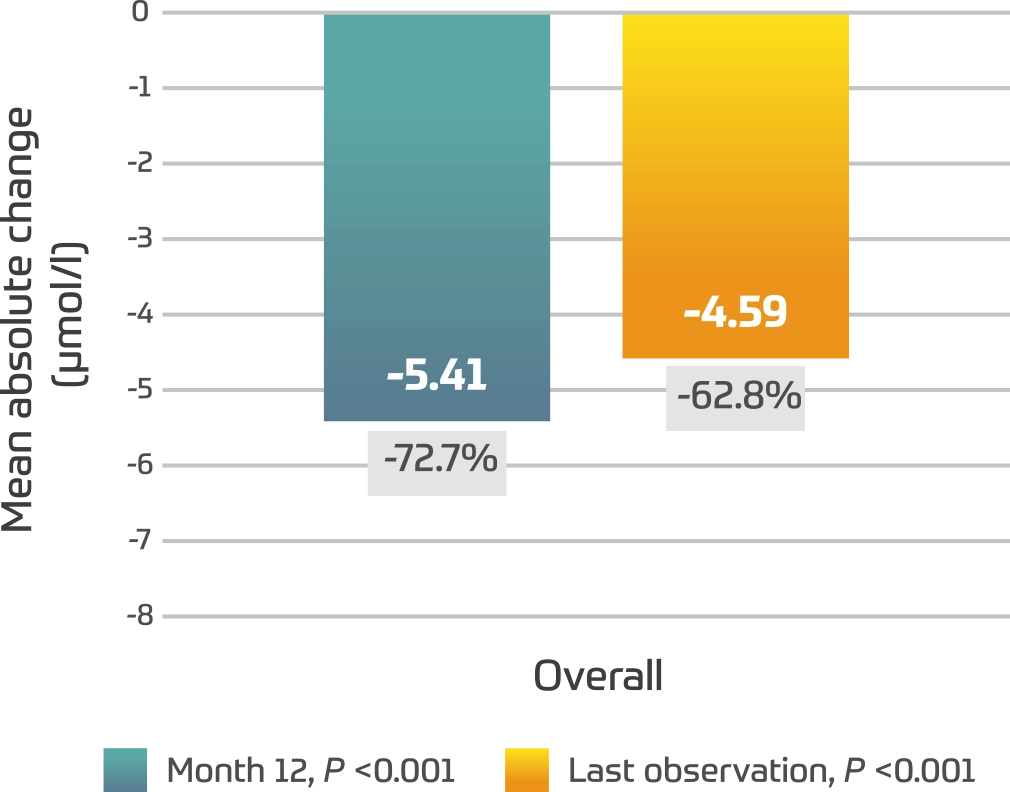
- Similar results were seen across age groups
3MSCT2
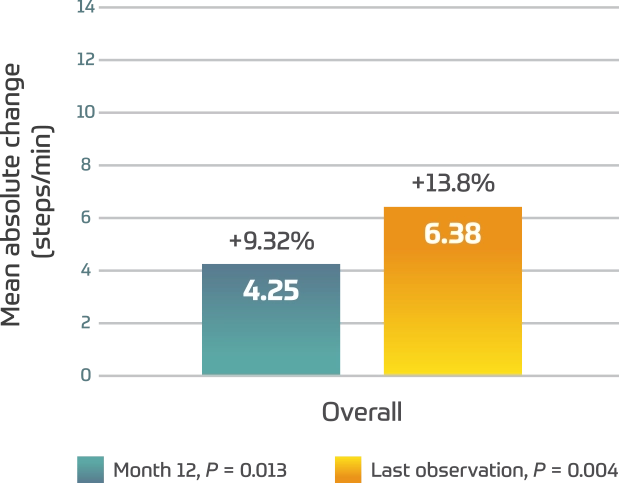
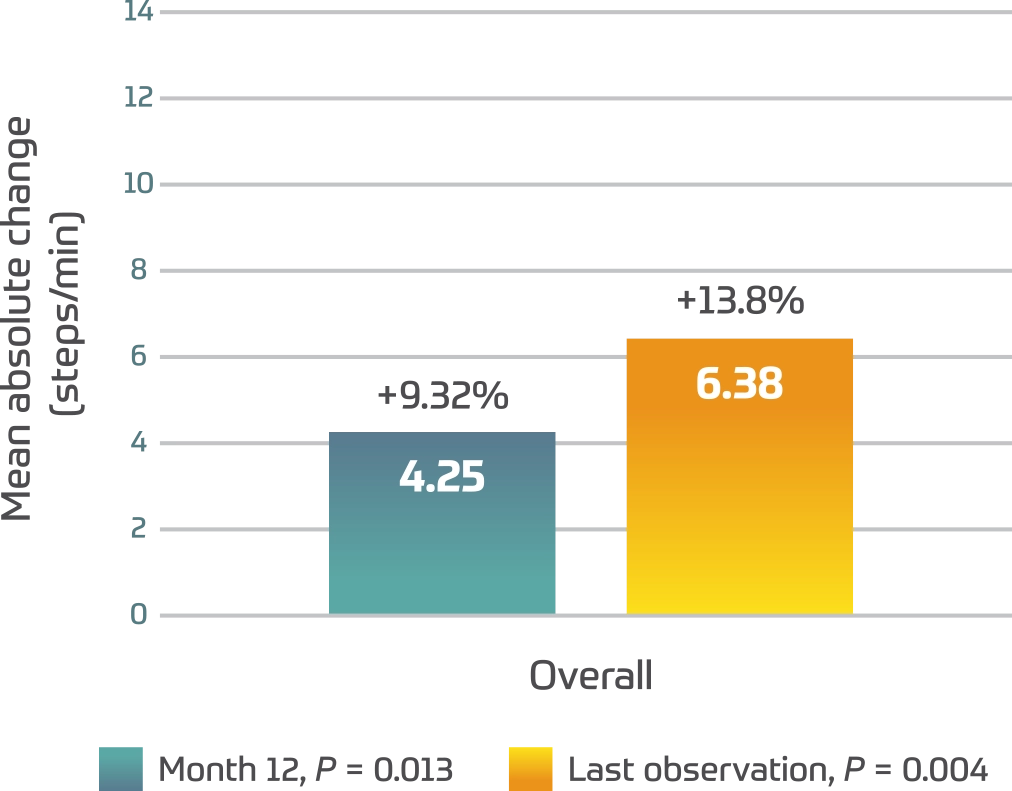
- A greater difference was seen in pediatric patients
| * | The integrated study design was necessary to assess long-term efficacy and safety in a progressively worsening disease despite the rarity of patient population. Although the placebo-control period was limited to 12 months, duration of follow-up and repeated assessments were designed to mitigate that limitation. Additionally, while the mean and median durations of exposure exceed 2 years, only a small proportion of patients in the developmental program have non-missing efficacy measurements after 18 months. Therefore, the ability of the integrated analysis to provide interpretable evidence of long-term efficacy is unclear. |
| † | One patient was originally in Phase I/II trials, but is only counted as part of Phase III. |
| ‡ | One patient was in the placebo group of Phase III pivotal trial, and continued in both the compassionate use program and an extension trial. Patient data is only counted once. |
| § | One patient was in the active Phase III pivotal trial, and continued in the compassionate use program, but did not participate in the long-term extension. Therefore, data represents 12 months. |