Lamzede has been thoroughly evaluated
in a 1-year trial
New treatments are approved based on their efficacy and safety—meaning, how well the drug works and the types of side effects people on the drug experience. In a key clinical trial, people taking Lamzede were compared to people taking a placebo (an intravenous solution without medicine).
Who was studied?
Lamzede can be used for people with alpha-mannosidosis of any age group or stage of development.
diagnosed with this ultra-rare disease
Lamzede,
including:
7 children (ages 6-17)
8 adults (18 or older)
placebo,
including:
5 children
5 adults
This study did not include people who previously had bone marrow transplants or who were unable to walk without support for shorter distances. People with other known genetic abnormalities and/or mental illness were also ineligible for the trial.

Lamzede was shown to:
Lower levels of oligosaccharides

Blood tests
Lamzede reduced a key marker of alpha-mannosidosis.
- Improvements at 6 months that continued at 1 year

75.8%
reduction of
oligosaccharides
with Lamzede
Relative difference
vs 20.3% reduction of oligosaccharides with placebo.
Preserve endurance climbing stairs
Functional tests
In a 3-minute stair-climbing test (3MSCT), people taking Lamzede maintained their endurance climbing stairs at 1 year, while people taking placebo got worse over time.*

in total number of steps over a year with Lamzede

in total number of steps over a year with placebo
Relative difference
*Differences in 3MSCT test were not statistically significant.
Results were sustained over time
Long-term data was collected for people between the ages of 6 and 35. The information was gathered from multiple studies and grouped together into what is called an integrated analysis.†
This study monitored people taking Lamzede for up to 4 years:
- The reduction in oligosaccharides was nearly as large at the end of this study as it was at 1 year
- People saw greater improvements in the 3MSCT with Lamzede at their last check-in than they did at 1 year
- On average, people were studied on Lamzede for 2 years and 5 months

†In the integrated analysis, placebo comparisons are not available after 1 year because those patients switched to the Lamzede treatment group. Despite length of treatment, not all studies measured each test at all follow-up visits, so more long-term research is necessary to understand results beyond 18 months.

In the key clinical trial, researchers also performed other tests to see how people functioned after taking Lamzede:

Lung function
In a forced vital capacity (FVC) test, people taking Lamzede showed greater improvements in a test of lung function than those taking placebo.
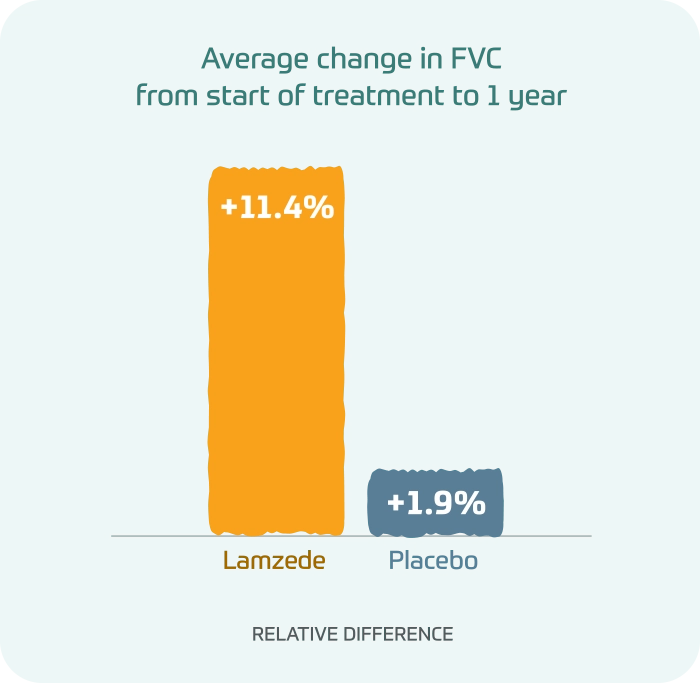
Average change in FVC from start of treatment to 1 year
- Lamzede: +11.4% relative difference
- Placebo: +1.9% relative difference
Differences in FVC test were not statistically significant.
Improvement in lung function with Lamzede

Walking distance
In a 6-minute walk test (6MWT), people taking Lamzede were able to walk farther at 1 year, while people taking placebo got worse over time.
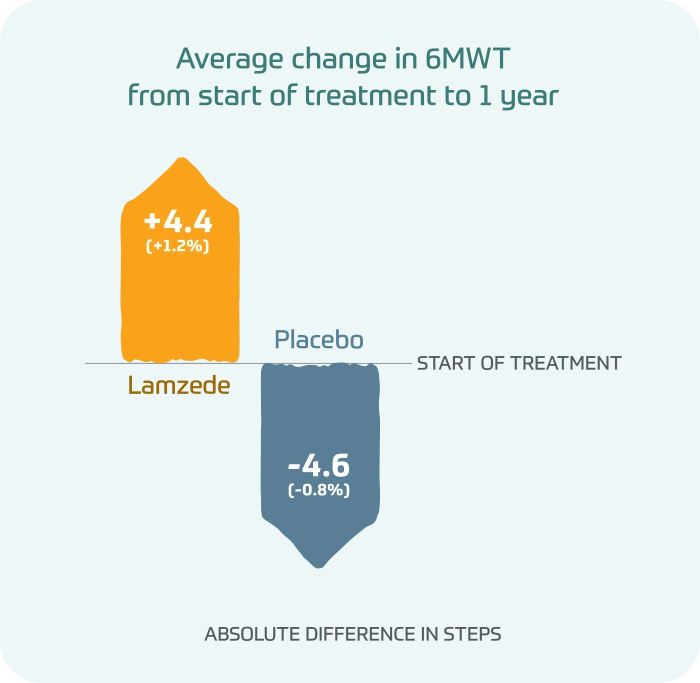
Average change in 6MWT from start of treatment to 1 year
- Lamzede: +4.4(+1.2%) absolute difference in steps
- Placebo: -4.6(-0.8%) absolute difference in steps
Differences in 6MWT were not statistically significant.
Lamzede may have the potential to help increase walking distance
Alpha-mannosidosis has no known cure, so maintaining long-term therapy is key for both children and adults.
Even if you aren’t seeing noticeable improvements, maintaining your current level of physical function and ability is an important goal when facing a progressive disease.
Balancing the potential benefits and adverse reactions of treatment
Every treatment has the potential to develop side effects while helping to achieve goals. Doctors carefully assess the benefits versus the risks when recommending treatment.
The most common adverse reactions in the 1-year clinical trial:
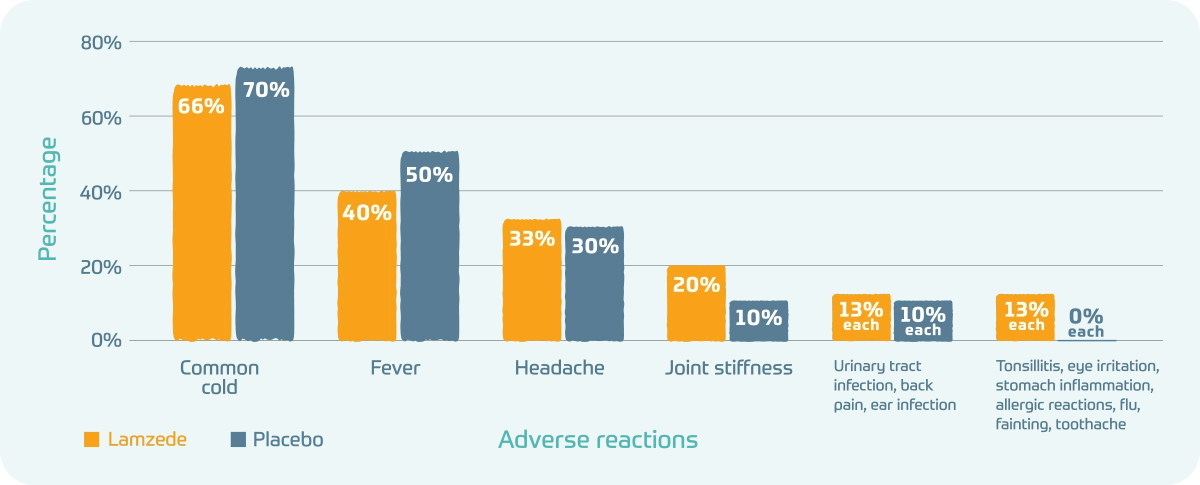
- Common cold: Lamzede 66%; Placebo 70%
- Fever: Lamzede 40%; Placebo 50%
- Headache: Lamzede 33%; Placebo 30%
- Joint stiffness: Lamzede 20%; Placebo 10%
- Urinary tract infection, back pain, ear infection: Lamzede 13% each; Placebo 10% each
- Tonsillitis, eye irritation, stomach inflammation, allergic reactions, flu, fainting, toothache: Lamzede 13% each; Placebo 0% each
In a long-term pediatric study:
One patient treated with Lamzede presented serious reactions (chills and hyperthermia on the same occasion).
- Additional adverse reactions that occurred in at least 2 of 5 patients included: cough, ear infection, congestion, pink eye, fall, ligament sprain, throat pain or infection, and facial swelling
And in a third long-term study:
Some people experienced upper abdominal pain, contusion, excoriation, post-lumbar puncture syndrome, wound, weight gain, erythema, rash, and tooth extraction.
If you notice any of these side effects, talk to a doctor. Continue with therapy unless otherwise directed
Your doctor may temporarily discontinue treatment if you have a severe hypersensitivity reaction (asthma attack, rash, pink eye, or anaphylactic shock) or a severe infusion-associated reaction.

Ask your doctor if Lamzede is right for you.